Neuralink Update – September 2023: First Parkinson's patient trial
Neuralink Update - September 2023: First Parkinson's patient trial
========
#neuralink #adamzackneuralink
In a remarkable stride towards technological innovation, Neuralink's N1 brain-machine interface trial is set to commence in September 2023, representing a pivotal moment in the field of neurology. This trial, involving 10 volunteers, holds the potential to reshape both neurological treatment and the lives of those grappling with Parkinson's disease, an ailment that has long eluded a definitive cure.
The article delves into the profound implications of the Neuralink N1 trial for Parkinson's disease treatment. It navigates through the trial's design, expected outcomes, and possible challenges, offering insights into how this groundbreaking technology could transform the landscape of Parkinson's care. The central question emerges: Can this wireless implant truly restore motor function and enhance the quality of life for affected individuals?
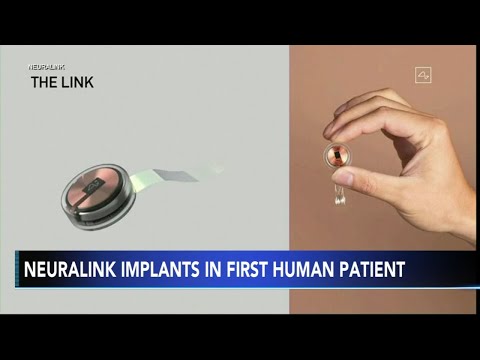
Neuralink's journey to this point has been guided by a rigorous process to address safety concerns. The device, implanted through a surgical robot, boasts wireless capabilities and houses 1024 electrodes distributed across 64 threads. The volunteers, encompassing diverse age groups, will have the device implanted in the motor cortex and spinal cord, enabling the recording and stimulation of motor-related neural activity.
A key distinction emerges when comparing Neuralink N1 to existing treatments. In contrast to deep brain stimulation (DBS), the N1 chip offers higher resolution, accuracy, and lower invasiveness. Additionally, the device's wireless nature and personalized approach stand out. When contrasted with gene therapy, Neuralink N1 presents advantages in terms of precision, resolution, and risk of adverse events. Similarly, in comparison to stem cell therapy, Neuralink N1 avoids challenges related to immune rejection, tumor formation, and ethical concerns.
Dr. Bastiaan Bloem, a prominent figure in neurology, highlights the complexity of Parkinson's and the limitations of current treatments. The Neuralink N1 trial emerges as an opportunity to address these limitations and potentially restore voluntary movement, thus enhancing the lives of those afflicted by Parkinson's. However, the trial's impact extends beyond individual treatment; it could illuminate novel insights into neural pathways, paving the way for customized neurotechnological solutions.
Amid this journey of advancement, active engagement is encouraged. The future of Parkinson's treatment and the broader field of neurology beckon exploration and dialogue. As Neuralink's N1 trial propels us into uncharted territories, the convergence of technology, medicine, and human ingenuity offers hope for a brighter future in neurology.
Tags: Neuralink N1 Trial, Parkinson's Disease Treatment, Brain-Machine Interface, Neurological Breakthrough, Medical Innovation, Neurotechnology Advancements, Parkinson's Research, Neural Pathways Exploration, Motor Function Restoration, Quality of Life Enhancement, Future of Neurology, Technological Advancement, Clinical Trials, Brain Implants, Medical Ethics.
Neuralink Update – September 2023: First Parkinson’s patient trial
========
#neuralink #adamzackneuralink
In a remarkable stride towards technological innovation, Neuralink’s N1 brain-machine interface trial is set to commence in September 2023, representing a pivotal moment in the field of neurology. This trial, involving 10 volunteers, holds the potential to reshape both neurological treatment and the lives of those grappling with Parkinson’s disease, an ailment that has long eluded a definitive cure.
The article delves into the profound implications of the Neuralink N1 trial for Parkinson’s disease treatment. It navigates through the trial’s design, expected outcomes, and possible challenges, offering insights into how this groundbreaking technology could transform the landscape of Parkinson’s care. The central question emerges: Can this wireless implant truly restore motor function and enhance the quality of life for affected individuals?
Neuralink’s journey to this point has been guided by a rigorous process to address safety concerns. The device, implanted through a surgical robot, boasts wireless capabilities and houses 1024 electrodes distributed across 64 threads. The volunteers, encompassing diverse age groups, will have the device implanted in the motor cortex and spinal cord, enabling the recording and stimulation of motor-related neural activity.
A key distinction emerges when comparing Neuralink N1 to existing treatments. In contrast to deep brain stimulation (DBS), the N1 chip offers higher resolution, accuracy, and lower invasiveness. Additionally, the device’s wireless nature and personalized approach stand out. When contrasted with gene therapy, Neuralink N1 presents advantages in terms of precision, resolution, and risk of adverse events. Similarly, in comparison to stem cell therapy, Neuralink N1 avoids challenges related to immune rejection, tumor formation, and ethical concerns.
Dr. Bastiaan Bloem, a prominent figure in neurology, highlights the complexity of Parkinson’s and the limitations of current treatments. The Neuralink N1 trial emerges as an opportunity to address these limitations and potentially restore voluntary movement, thus enhancing the lives of those afflicted by Parkinson’s. However, the trial’s impact extends beyond individual treatment; it could illuminate novel insights into neural pathways, paving the way for customized neurotechnological solutions.
Amid this journey of advancement, active engagement is encouraged. The future of Parkinson’s treatment and the broader field of neurology beckon exploration and dialogue. As Neuralink’s N1 trial propels us into uncharted territories, the convergence of technology, medicine, and human ingenuity offers hope for a brighter future in neurology.
Tags: Neuralink N1 Trial, Parkinson’s Disease Treatment, Brain-Machine Interface, Neurological Breakthrough, Medical Innovation, Neurotechnology Advancements, Parkinson’s Research, Neural Pathways Exploration, Motor Function Restoration, Quality of Life Enhancement, Future of Neurology, Technological Advancement, Clinical Trials, Brain Implants, Medical Ethics.